Abstract
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired disease, characterized by chronic intravascular hemolysis and increased risk of thrombosis. Pozelimab is an investigational fully human monoclonal antibody inhibitor of complement component C5. A healthy volunteer study (NCT03115996) demonstrated that pozelimab was generally well tolerated while providing complete inhibition of ex-vivo-assessed hemolytic activity. This led to the first-in-patient study of pozelimab monotherapy in participants with PNH. Here we present the final results from a phase 2, open-label, single-arm study (NCT03946748), and the subsequent open-label extension (OLE) study (NCT04162470), evaluating the long-term efficacy and safety of pozelimab monotherapy in patients with PNH.
Methods: The phase 2, 26-week study enrolled 24 patients with active symptomatic PNH who were naïve to complement inhibitor therapy, or who had received prior treatment with a complement inhibitor (>6 months prior to the start of the study). Patients received a single intravenous loading dose of pozelimab (30 mg/kg), followed by a weekly subcutaneous (SC) dose of 800 mg through to Week 26. All patients who completed the phase 2 study were eligible to enroll in the OLE study, and were maintained on weekly SC pozelimab 800 mg up to Week 104. The effects of pozelimab on intravascular hemolysis (monitored via lactate dehydrogenase [LDH] levels), transfusion avoidance, and safety were assessed from baseline to Week 26 during the phase 2 trial. Long-term efficacy and safety of pozelimab monotherapy was assessed up to Week 104 in the OLE study.
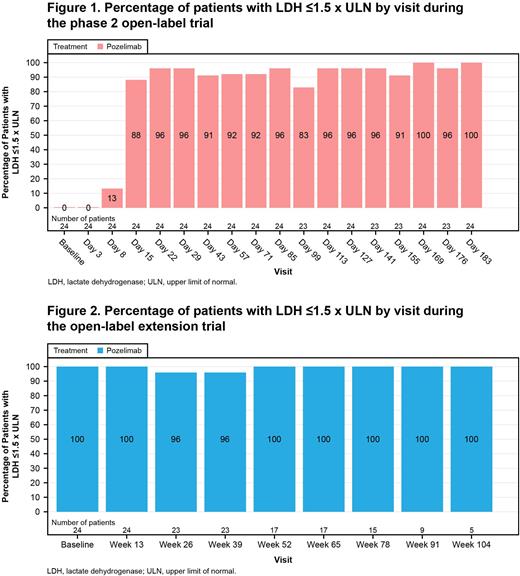
Results: All 24 patients completed the phase 2 study and enrolled into the OLE study. Most patients were Asian (87.5%) and over half were male (54.2%). During the phase 2 study, treatment with pozelimab led to rapid and sustained reduction in LDH through Week 26. At every scheduled time point between Weeks 4 through 26 inclusive, a total of 18/24 (75.0%) patients achieved adequate control of their intravascular hemolysis (defined as LDH ≤1.5 x upper limit of normal [ULN], 95% confidence interval [CI]: 57.7-92.3%). During the OLE trial, 23 (95.8%) and 15 (62.5%) patients completed treatment through to Week 26 and Week 78, respectively. Control of intravascular hemolysis was achieved by 22/23 (95.7%) patients at all time points through the initial 26 weeks of the OLE trial (95% CI: 87.3-100.0%); for Week 78, the results are 15/16 (93.8%; 95% CI: 81.9-100.0%). At each study visit, 83-100% of patients achieved adequate control of hemolysis for both the phase 2 and the OLE trials (Figures 1 and 2).
Additionally, during the 26-week phase 2 trial, 21/24 (87.5%; 95% CI: 74.3-100.0%) patients achieved transfusion avoidance (defined as no post-baseline transfusion of red blood cells). Similarly, during the OLE, 22/23 (95.7%) patients achieved transfusion avoidance through the initial 26 weeks of the extension period, and 15/16 (93.8%) through week 78. No patients experienced a breakthrough hemolysis event in either trial. Importantly, one patient with a C5 variant known to be resistant to blockade by eculizumab/ravulizumab had adequate control of intravascular hemolysis and achieved transfusion avoidance. From Day 1, all patients showed a rapid decrease in total complement hemolytic activity (CH50) to near zero; median CH50 values were maintained at 0 up to Week 104.
During the phase 2 trial, 21 patients (87.5%) experienced a total of 72 treatment-emergent adverse events (TEAEs). Two patients (8.3%) experienced severe TEAEs, and 10 patients (41.7%) experienced TEAEs considered related to pozelimab treatment. During the OLE, 15 patients (62.5%) experienced a total of 41 TEAEs; two patients (8.3%) experienced serious TEAEs, two patients (8.3%) experienced three severe TEAEs, and two patients (8.3%) experienced TEAEs considered related to pozelimab treatment. No deaths or discontinuations due to TEAEs occurred during the OLE.
Conclusions: Patients treated with pozelimab (up to 130 weeks) experienced improvement in control of their intravascular hemolysis, saw no breakthrough hemolysis, and were able to achieve transfusion avoidance. Pozelimab was also generally well tolerated. The data suggests that pozelimab may be an effective long-term treatment for patients with PNH.
Disclosures
Wong:F. Hoffmann-La Roche AG: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis Pharmaceuticals: Research Funding, Speakers Bureau. Weyne:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Chaudhari:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Miller:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Dain:Regeneron Pharmaceuticals, Inc.: Current Employment. Griffin:Alexion: Honoraria, Other: Conference support; BioCryst Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Sobi Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: educational work sponsored by Apellis with unrestricted grant paid to Medscape.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal